Multiple Choice
Consider the reaction Cu2+(aq) + 4 NH3(aq) 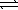 Cu(NH3) 42+(aq)
Cu(NH3) 42+(aq)
Kf = 2.1 × 1013
If the Ksp for Cu(OH) 2 is 2.2 × 10-20,what is the value of the equilibrium constant,K,for the reaction below?
Cu(NH3) 42+(aq) + 2 OH-(aq) 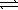 Cu(OH) 2(s) + 4 NH3(aq)
Cu(OH) 2(s) + 4 NH3(aq)
A) 1.0 × 10-33
B) 4.6 × 10-7
C) 2.1 × 1013
D) 2.2 × 106
E) 9.5 × 1032
Correct Answer:

Verified
Correct Answer:
Verified
Q36: A 50.00-mL solution of 0.0350 M ethylamine
Q37: What mass of solid NaCH<sub>3</sub>CO<sub>2</sub> (molar mass
Q38: Hyperventilation can cause your blood pH to
Q39: What is the pH of an aqueous
Q40: A 1.0-liter solution contains 0.25 M HF
Q42: A solution containing 10.mmol of CO<sub>3</sub><sup>2</sup><sup>−</sup>and 5.0
Q43: An impure sample of sodium carbonate,Na<sub>2</sub>CO<sub>3</sub>,is titrated
Q44: A 25.00-mL sample of propionic acid,HC<sub>3</sub>H<sub>5</sub>O<sub>2</sub>,of unknown
Q45: To make a buffer with a pH
Q46: What is the pH of a saturated