Multiple Choice
Which equation depicts aqueous hydrogen sulfide behaving as a Brønsted-Lowry base in water?
A) H2S(aq) + 2 OH-(aq) 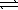 SO2(aq) + 2 H2(g)
SO2(aq) + 2 H2(g)
B) H2S(aq) + H3O+(aq) 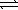 H3S+(aq) + H2O(
H3S+(aq) + H2O( 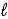 )
)
C) H2S(aq) + H2O( 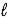 )
) 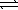 HS-(aq) + H3O+(aq)
HS-(aq) + H3O+(aq)
D) HS-(aq) + H3O+(aq) 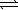 H2S(aq) + H2O(
H2S(aq) + H2O( 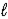 )
)
E) H2S(aq) + H2O( 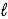 )
) 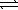 H3S++(aq) + OH-(aq)
H3S++(aq) + OH-(aq)
Correct Answer:

Verified
Correct Answer:
Verified
Q13: Which of the following statements is INCORRECT?<br>A)
Q14: Which acid-base reaction results in acidic solution?<br>A)
Q15: What is the equilibrium pH of a
Q16: Calculate the pH of the following aqueous
Q17: The H<sub>3</sub>O<sup>+</sup> concentration of a solution is
Q19: What volume of water must be added
Q20: Which of the following equations shows that
Q21: What is the OH<sup>-</sup> concentration of an
Q22: Which of the following molecules or ions
Q23: What is the pH of the solution