Multiple Choice
Which acid-base reaction results in acidic solution?
A) HBr(aq) + KOH(aq) → H2O( 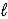 ) + KBr(aq)
) + KBr(aq)
B) HNO3(aq) + LiOH(aq) → H2O( 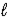 ) + LiNO3(aq)
) + LiNO3(aq)
C) HCl(aq) + KOH(aq) → H2O( 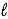 ) + KCl(aq)
) + KCl(aq)
D) H2SO4(aq) + CsOH(aq)  H2O(
H2O( 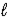 ) + CsHSO4(aq)
) + CsHSO4(aq)
E) HF(aq) + RbOH(aq) 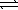 H2O(
H2O( 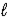 ) + RbF(aq)
) + RbF(aq)
Correct Answer:

Verified
Correct Answer:
Verified
Q9: What is the conjugate base of HPO<sub>4</sub><sup>3-</sup>
Q10: What is the equilibrium constant for the
Q11: Which of the following ionic compounds forms
Q13: Which of the following statements is INCORRECT?<br>A)
Q15: What is the equilibrium pH of a
Q16: Calculate the pH of the following aqueous
Q17: The H<sub>3</sub>O<sup>+</sup> concentration of a solution is
Q18: Which equation depicts aqueous hydrogen sulfide behaving
Q19: What volume of water must be added
Q51: Which of the following is the strongest