Multiple Choice
What is the equilibrium constant for the following reaction, HCO2H(aq) + CN-(aq) 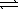 HCO2-(aq) + HCN(aq)
HCO2-(aq) + HCN(aq)
And does the reaction favor the formation of reactants or products? The acid dissociation constant,Ka,for HCO2H is 1.8 × 10-4 and the acid dissociation constant for HCN is 4.0 × 10-10.
A) K = 1.00.The reaction favors neither the formation of reactants nor products.
B) K = 2.2 × 10-6.The reaction favors the formation of products.
C) K = 2.2 × 10-6.The reaction favors the formation of reactants.
D) K = 4.5 × 105.The reaction favors the formation of products.
E) K = 4.5 × 105.The reaction favors the formation of reactants.
Correct Answer:

Verified
Correct Answer:
Verified
Q5: Which of the following species is amphiprotic
Q6: Which equation depicts the hydrogen phosphate ion
Q7: What is the pOH of 0.074 M
Q8: What is the pH of a solution
Q9: What is the conjugate base of HPO<sub>4</sub><sup>3-</sup>
Q11: Which of the following ionic compounds forms
Q13: Which of the following statements is INCORRECT?<br>A)
Q14: Which acid-base reaction results in acidic solution?<br>A)
Q15: What is the equilibrium pH of a
Q51: Which of the following is the strongest