Multiple Choice
Given the equilibrium constants for the equilibria, H2O(l) 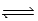 H3O+(aq) ; K =
H3O+(aq) ; K = 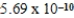 2CH3COOH(aq) + 2H2O(l)
2CH3COOH(aq) + 2H2O(l) 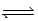 2CH3COO−(aq) + 2H3O+(aq) ; K =
2CH3COO−(aq) + 2H3O+(aq) ; K = 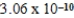 determine Kc for the following equilibrium.
determine Kc for the following equilibrium.
CH3COOH(aq) + NH3(aq) 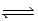 CH3COO−(aq) + NH4+(aq)
CH3COO−(aq) + NH4+(aq)
A) 3.08 × 104
B) 3.25 × 10-5
C) 9.96 × 10-15
D) 1.00 × 1014
E) 1.75 × 10-5
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q22: Which of the following molecules or ions
Q23: What is the pH of the solution
Q24: An aqueous solution with a pH of
Q25: What is the equilibrium pH of an
Q26: A molecule that can behave as either
Q28: Which of the following statements about the
Q29: What is the pH of a 0.046
Q30: Which of the following species is the
Q31: What is the pH of a solution
Q32: Write a net ionic equation for the