Multiple Choice
What is the equilibrium pH of an initially 0.83 M solution of the monoprotic acid 3-chloropropanoic acid at 25°C (Ka = 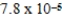 ) ?
) ?
A) 2.10
B) 7.00
C) 1.79
D) 12.21
E) 5.21
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q20: Which of the following equations shows that
Q21: What is the OH<sup>-</sup> concentration of an
Q22: Which of the following molecules or ions
Q23: What is the pH of the solution
Q24: An aqueous solution with a pH of
Q26: A molecule that can behave as either
Q27: Given the equilibrium constants for the equilibria,
Q28: Which of the following statements about the
Q29: What is the pH of a 0.046
Q30: Which of the following species is the