Multiple Choice
For which of the following reactions is Kc = Kp?
A) N2(g) + 3 H2(g) 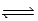 2 NH3(g)
2 NH3(g)
B) CO(g) + H2O(g) 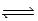 CO2(g) + H2(g)
CO2(g) + H2(g)
C) CO(g) + 3 H2(g) 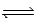 CH4(g) + H2O(g)
CH4(g) + H2O(g)
D) CaO(s) + CO2(g) 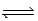 CaCO3(s)
CaCO3(s)
E) HBr(g) 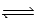 ½ H2(g) + ½ Br2(
½ H2(g) + ½ Br2( 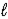 )
)
Correct Answer:

Verified
Correct Answer:
Verified
Q50: In an experiment,0.46 mol H<sub>2</sub> and 0.46
Q51: At 25 °C,the decomposition of dinitrogen tetraoxide
Q52: An aqueous mixture of phenol and ammonia
Q53: Given the following chemical equilibria, N<sub>2</sub>(g)+ O<sub>2</sub>(g)
Q54: For the equilibrium PCl<sub>5</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7480/.jpg" alt="For
Q56: Given the following chemical equilibrium COCl<sub>2</sub>(g) <img
Q57: Exactly 1.0 mol N<sub>2</sub>O<sub>4</sub> is placed in
Q58: If the reaction quotient,Q,is greater than K
Q59: If a stress is applied to an
Q60: Consider the reaction H<sub>2</sub> + I<sub>2</sub> <img