Multiple Choice
At 25 °C,the decomposition of dinitrogen tetraoxide N2O4(g) 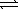 2 NO2(g)
2 NO2(g)
Has an equilibrium constant (Kp) of 0.144.At equilibrium,the total pressure of the system is 0.0758 atm.What is the partial pressure of each gas?
A) 0.0745 atm NO2(g) and 0.0385 N2O4(g)
B) 0.0549 atm NO2(g) and 0.0209 N2O4(g)
C) 0.0531 atm NO2(g) and 0.0227 N2O4(g)
D) 0.0502 atm NO2(g) and 0.0256 N2O4(g)
E) 0.0381 atm NO2(g) and 0.0377 N2O4(g)
Correct Answer:

Verified
Correct Answer:
Verified
Q46: Sulfuryl chloride decomposes to sulfur dioxide and
Q47: Which of the following expressions correctly describes
Q48: The equilibrium constant at 25 °C for
Q49: Which of the following statements about the
Q50: In an experiment,0.46 mol H<sub>2</sub> and 0.46
Q52: An aqueous mixture of phenol and ammonia
Q53: Given the following chemical equilibria, N<sub>2</sub>(g)+ O<sub>2</sub>(g)
Q54: For the equilibrium PCl<sub>5</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7480/.jpg" alt="For
Q55: For which of the following reactions is
Q56: Given the following chemical equilibrium COCl<sub>2</sub>(g) <img