Multiple Choice
The reaction quotient,Q,for a system is 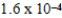 .If the equilibrium constant for the system at some temperature is
.If the equilibrium constant for the system at some temperature is 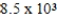 ,what will happen as the reaction mixture returns to equilibrium?
,what will happen as the reaction mixture returns to equilibrium?
A) The equilibrium constant will increase until it equals the reaction quotient.
B) There will be a net loss in both product(s) and reactant(s) .
C) There will be a net loss in product(s) .
D) There will be a net loss in reactant(s) .
E) The equilibrium constant will increase.
Correct Answer:

Verified
Correct Answer:
Verified
Q2: Which of the following expressions for K
Q3: Given the equilibrium constants for the following
Q4: What is the expression for K<sub>c</sub> for
Q5: A 2.5 L flask is filled with
Q6: For the reaction given below,2.00 moles of
Q8: When 1.0 mole of acetic acid is
Q9: Which of the following is the correct
Q10: If the reaction quotient,Q,is equal to K
Q11: Which of the following is always true
Q12: In which of the following reactions does