Multiple Choice
Given the equilibrium constants for the following reactions: 4Cu(s) + O2(g)  2Cu2O(s) ,K1
2Cu2O(s) ,K1
4CuO(s)  2Cu2O(s) + O2(g) ,K2
2Cu2O(s) + O2(g) ,K2
What is K for the system
2Cu(s) + O2(g)  2CuO(s)
2CuO(s)
Equivalent to?
A) 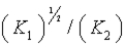
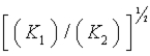
B) 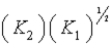
C) (K1) (K2)
D) 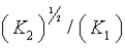
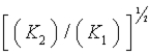
E) 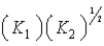
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: What is the K<sub>c</sub> equilibrium-constant expression for
Q2: Which of the following expressions for K
Q4: What is the expression for K<sub>c</sub> for
Q5: A 2.5 L flask is filled with
Q6: For the reaction given below,2.00 moles of
Q7: The reaction quotient,Q,for a system is <img
Q8: When 1.0 mole of acetic acid is
Q9: Which of the following is the correct
Q10: If the reaction quotient,Q,is equal to K
Q11: Which of the following is always true