Multiple Choice
At 700 K,Kp for the following equilibrium is 5.6 × 10-3. 2HgO(s) 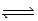 2Hg(l) + O2(g)
2Hg(l) + O2(g)
Suppose 43.1 g of mercury(II) oxide is placed in a sealed 4.00-L vessel at 700 K.What is the partial pressure of oxygen gas at equilibrium? (R = 0.0821 L · atm/(K · mol) )
A) 0.074 atm
B) 0.0056 atm
C) 2.8 atm
D) 14 atm
E) 1.4 atm
Correct Answer:

Verified
Correct Answer:
Verified
Q39: Given the following equilibria, Ni<sup>2+</sup>(aq)+ 2 OH<sup>-</sup>(aq)
Q40: Consider the following equilibria. PbBr<sub>2</sub>(s) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7480/.jpg"
Q41: Which of the following equilibria would not
Q42: Nitrosyl bromide decomposes according to the chemical
Q43: At 800 K,the equilibrium constant,K<sub>p</sub>,for the following
Q45: For the reaction N<sub>2</sub>O<sub>4</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7480/.jpg" alt="For
Q46: Sulfuryl chloride decomposes to sulfur dioxide and
Q47: Which of the following expressions correctly describes
Q48: The equilibrium constant at 25 °C for
Q49: Which of the following statements about the