Multiple Choice
Nitrogen and oxygen gases may react to form nitrogen monoxide.At 1500 °C,Kc equals 1.0 × 10−5. N2(g) + O2(g) 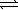 2 NO(g)
2 NO(g)
If 0.030 mol N2 and 0.030 mol O2 are sealed in a 1.0 L flask at 1500 °C,what is the concentration of NO(g) when equilibrium is established?
A) 3.0 × 10−7 M
B) 4.7 × 10−5 M
C) 9.5 × 10−5 M
D) 3.0 × 10−2 M
E) 9.1 × 101 M
Correct Answer:

Verified
Correct Answer:
Verified
Q33: Which of the following is the correct
Q34: A 3.00-liter flask initially contains 3.00 mol
Q35: At a high temperature,equal concentrations of 0.160
Q36: For which of the following reactions is
Q37: Given the equilibrium constants for the equilibria,
Q39: Given the following equilibria, Ni<sup>2+</sup>(aq)+ 2 OH<sup>-</sup>(aq)
Q40: Consider the following equilibria. PbBr<sub>2</sub>(s) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7480/.jpg"
Q41: Which of the following equilibria would not
Q42: Nitrosyl bromide decomposes according to the chemical
Q43: At 800 K,the equilibrium constant,K<sub>p</sub>,for the following