Multiple Choice
The Arrhenius equation, 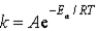 ,relates the rate constant of reaction and temperature.A plot of ____ versus 1/T will yield a straight line with a slope of -Ea/R.
,relates the rate constant of reaction and temperature.A plot of ____ versus 1/T will yield a straight line with a slope of -Ea/R.
A) k2/k1
B) -Ea
C) ln(k)
D) 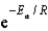
E) 1/RT
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q45: For which of the following hypothetical rate
Q46: What is the overall order of the
Q47: The rate law for a reaction is
Q48: Which of the following is not a
Q49: A student analyzed a first-order reaction and
Q51: How many mechanistic steps are depicted by
Q51: Below is a proposed mechanism for the
Q52: The rate constant at 366 K for
Q53: For the second-order reaction below,the initial concentration
Q54: The rate constant for a first-order reaction