Multiple Choice
What is the overall order of the reaction NO(g) + O3(g) → NO2(g) + O2(g) if the reaction proceeds via the rate expression given below. 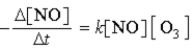
A) Zero-order
B) First-order
C) Second-order
D) Third-order
E) Fourth-order
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q41: Which of the given relationships correctly compares
Q42: For the overall reaction 2A + B
Q43: Which of the following statements is true
Q44: The reaction A → B follows first-order
Q45: For which of the following hypothetical rate
Q47: The rate law for a reaction is
Q48: Which of the following is not a
Q49: A student analyzed a first-order reaction and
Q50: The Arrhenius equation, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7480/.jpg" alt="The Arrhenius
Q51: How many mechanistic steps are depicted by