Multiple Choice
Which of the following statements is true of the Arrhenius equation?
A) The factor 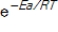 always has a value more than 1.
always has a value more than 1.
B) Ea represents the fraction of molecules having the minimum energy required for a reaction.
C) It can be used to calculate Ea from the temperature dependence of the rate constant.
D) It can be used to calculate the rate constant if 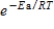 is known.
is known.
E) The factor 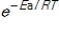 always has a value more than 10.
always has a value more than 10.
Correct Answer:

Verified
Correct Answer:
Verified
Q38: The decomposition of phosphine,PH<sub>3</sub>,follows first-order kinetics. 4
Q39: Nitrogen dioxide reacts with carbon monoxide to
Q40: The _ of an elementary step is
Q41: Which of the given relationships correctly compares
Q42: For the overall reaction 2A + B
Q44: The reaction A → B follows first-order
Q45: For which of the following hypothetical rate
Q46: What is the overall order of the
Q47: The rate law for a reaction is
Q48: Which of the following is not a