Multiple Choice
Given the accompanying phase diagram,under what conditions will liquid be found in equilibrium with either solid or gas? 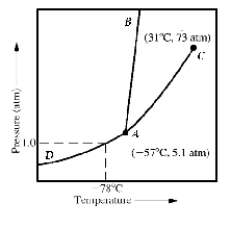
A) Anywhere along curve AB
B) Anywhere along curve AC
C) Anywhere along curve AD
D) Anywhere along curve AB and AC
E) Anywhere along curve AB and AD
Correct Answer:

Verified
Correct Answer:
Verified
Q20: A sketch of a phase diagram is
Q21: Which of the compounds below is not
Q22: In what type of unit cell are
Q23: The space-filling representation provided below is an
Q24: A metal crystallizes in a face-centered cubic
Q26: An unknown white solid was found to
Q27: Palladium crystallizes in a face-centered cubic lattice
Q28: Chromium (atomic mass 52.00 g/mol)crystallizes in a
Q29: Which one of the following elements is
Q30: Silver chloride adopts the sodium chloride (rock