Multiple Choice
A sketch of a phase diagram is given below. 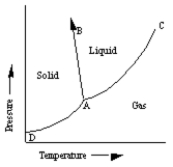 Which statement about this diagram is not true?
Which statement about this diagram is not true?
A) Increasing pressure at constant temperature can melt the solid.
B) Increasing temperature at constant pressure can cause the solid to sublime.
C) Increasing temperature at constant pressure can cause the liquid to vaporize.
D) Increasing pressure at constant temperature can cause deposition of solid from gas.
E) Increasing pressure at constant temperature can cause liquid to freeze.
Correct Answer:

Verified
Correct Answer:
Verified
Q15: The bandgap of ZnTe is 218 kJ/mol.What
Q16: The metal sodium crystallizes in a body-centered
Q17: If an ionic compound with the formula
Q18: In metals,there are not enough electrons to
Q19: Which one of the following statements is
Q21: Which of the compounds below is not
Q22: In what type of unit cell are
Q23: The space-filling representation provided below is an
Q24: A metal crystallizes in a face-centered cubic
Q25: Given the accompanying phase diagram,under what conditions