Multiple Choice
How many sigma and pi bonds are in the molecule pictured below? 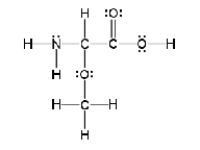
A) thirteen sigma bonds and one pi bond
B) eleven sigma bonds and two pi bonds
C) thirteen sigma bonds and two pi bonds
D) eleven sigma bonds and five pi bonds
E) five sigma bonds and eleven pi bonds
Correct Answer:

Verified
Correct Answer:
Verified
Q3: Which of the underlined atoms (C<sub>1</sub>,C<sub>2</sub>,N,and O)are
Q4: In valence bond theory,each sigma bond in
Q5: What is the hybridization of oxygen atom
Q6: What is the molecular geometry around an
Q7: A molecular orbital that decreases the electron
Q9: How many sigma (σ)bonds and pi (π)bonds
Q10: For which of the following molecules or
Q11: Refer to Diagram 9-1.According to molecular orbital
Q12: What does the following figure represent? <img
Q13: What is the hybridization of carbon atoms