Multiple Choice
Which of the underlined atoms (C1,C2,N,and O) are sp2 hybridized? 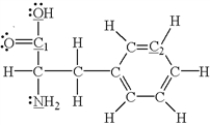
A) N and O
B) C1 and N
C) C1 and O
D) C2 and N
E) C1 and C2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: What is the hybridization of the central
Q2: For which of the following molecules and
Q4: In valence bond theory,each sigma bond in
Q5: What is the hybridization of oxygen atom
Q6: What is the molecular geometry around an
Q7: A molecular orbital that decreases the electron
Q8: How many sigma and pi bonds are
Q9: How many sigma (σ)bonds and pi (π)bonds
Q10: For which of the following molecules or
Q11: Refer to Diagram 9-1.According to molecular orbital