Multiple Choice
How many orbitals have the following set of quantum numbers: n = 5, 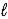 = 3,
= 3, 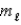 = -1?
= -1?
A) 0
B) 1
C) 3
D) 6
E) 7
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q29: In Bohr's atomic theory,when an electron moves
Q55: A device emits light at 219.4 nm.What
Q56: A point in a standing wave that
Q57: Occupied states or energy levels in the
Q58: What evidence does the photoelectric effect provide
Q59: What is the wavelength of light emitted
Q62: The (principal quantum number)n = _ shell
Q63: For which of the following transitions would
Q64: What type of orbital is designated n
Q65: If the energy of 1.00 mole of