Multiple Choice
What type of orbital is designated n = 4, 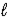 = 2,
= 2, 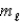 = 2?
= 2?
A) 4f
B) 4d
C) 4p
D) 4g
E) 4s
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q29: In Bohr's atomic theory,when an electron moves
Q55: A device emits light at 219.4 nm.What
Q56: A point in a standing wave that
Q57: Occupied states or energy levels in the
Q58: What evidence does the photoelectric effect provide
Q59: What is the wavelength of light emitted
Q61: How many orbitals have the following set
Q62: The (principal quantum number)n = _ shell
Q63: For which of the following transitions would
Q65: If the energy of 1.00 mole of