Multiple Choice
Which of the following sets of quantum numbers refers to a 4d orbital?
A) n = 2, 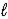 = 1,
= 1, 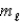 = -1
= -1
B) n = 2,  = 4,
= 4, 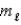 = -1
= -1
C) n = 4, 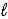 = 2,
= 2, 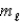 = -1
= -1
D) n = 4, 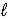 = 3,
= 3, 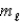 = 0
= 0
E) n = 4, 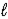 = 3,
= 3, 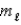 = +2
= +2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q12: What is the value of the spin
Q13: Which of the following orbital boundary surfaces
Q14: What is the energy of a photon
Q15: Which of the following orbital boundary surfaces
Q16: For a proton (mass = 1.673 ×
Q18: What is the frequency of gamma ray
Q19: Which type of experiment demonstrates that light
Q20: A 4d orbital has ?<br>A) 3 planar
Q21: A red laser pointer emits light at
Q22: A light emitting diode (L.E.D.)emits photons with