Multiple Choice
What is the value of the spin quantum number for an electron in a 3d orbital?
A) 3
B) 2
C) either 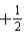 or
or 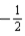
D) 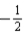
E) 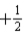
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q7: For which of the following transitions would
Q8: Which of the following statements is INCORRECT?<br>A)
Q9: What is the total number of subshells
Q10: The electron in a hydrogen atom,originally in
Q11: A _,designated by the Greek symbol ψ,describes
Q13: Which of the following orbital boundary surfaces
Q14: What is the energy of a photon
Q15: Which of the following orbital boundary surfaces
Q16: For a proton (mass = 1.673 ×
Q17: Which of the following sets of quantum