Multiple Choice
What quantity,in moles,of hydrogen is consumed when 179.6 kJ of energy is evolved from the combustion of a mixture of H2(g) and O2(g) ? H2(g) + 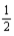 O2(g) → H2O(l) ; ΔrH° = -285.8 kJ/mol-rxn
O2(g) → H2O(l) ; ΔrH° = -285.8 kJ/mol-rxn
A) 0.6284 mol
B) 0.3142 mol
C) 1.591 mol
D) 1.628 mol
E) 0.3716 mol
Correct Answer:

Verified
Correct Answer:
Verified
Q54: Determine the enthalpy change for the decomposition
Q55: How much heat is liberated at constant
Q56: Commercial cold packs consist of solid ammonium
Q57: When 50.0 mL of 1.60 M of
Q58: The energy associated with a stretched spring
Q60: A 170.0-g sample of metal at 83.00°C
Q61: In thermodynamics,a(n)_ is defined as the object,or
Q62: At constant pressure and 25°C,what is Δ<sub>r</sub>H°
Q63: If 50.0 g of benzene (C<sub>6</sub>H<sub>6</sub>)absorbs 2.71
Q64: A 18.6-g piece of gold (s =