Multiple Choice
When 50.0 mL of 1.60 M of HCl(aq) is combined with 50.0 mL of 1.70 M of NaOH(aq) in a coffee-cup calorimeter,the temperature of the solution increases by 10.7°C.What is the change in enthalpy for this balanced reaction? HCl(aq) + NaOH(aq) → NaCl(aq) + H2O( 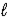 )
)
Assume that the solution density is 1.00 g/mL and the specific heat capacity of the solution is 4.18 J/g⋅°C.
A) -55.8 kJ
B) 55.8 kJ
C) 52.5 kJ
D) -52.5 kJ
E) -27.1 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Q52: Which of the following has zero standard
Q53: Combustion of 2.14 g of liquid benzene
Q54: Determine the enthalpy change for the decomposition
Q55: How much heat is liberated at constant
Q56: Commercial cold packs consist of solid ammonium
Q58: The energy associated with a stretched spring
Q59: What quantity,in moles,of hydrogen is consumed when
Q60: A 170.0-g sample of metal at 83.00°C
Q61: In thermodynamics,a(n)_ is defined as the object,or
Q62: At constant pressure and 25°C,what is Δ<sub>r</sub>H°