Multiple Choice
Combustion of 2.14 g of liquid benzene (C6H6) causes a temperature rise of 16.2 °C in a constant-pressure calorimeter that has a heat capacity of 5.53 kJ/°C.What is ΔH for the following reaction? C6H6(l) + 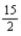 O2(g) → 6 CO2(g) + 3 H2O(
O2(g) → 6 CO2(g) + 3 H2O( 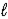 )
)
A) -89.5 kJ/mol-rxn
B) 41.8 kJ/mol-rxn
C) -41.8 kJ/mol-rxn
D) -3.27 × 103 kJ/mol-rxn
E) 89.5 kJ/mol-rxn
Correct Answer:

Verified
Correct Answer:
Verified
Q48: When 1 mole of Fe<sub>2</sub>O<sub>3</sub>(s)reacts with H<sub>2</sub>(g)to
Q49: Specific heat capacity is<br>A) the quantity of
Q50: How much energy is needed to convert
Q51: Calculate the energy in the form of
Q52: Which of the following has zero standard
Q54: Determine the enthalpy change for the decomposition
Q55: How much heat is liberated at constant
Q56: Commercial cold packs consist of solid ammonium
Q57: When 50.0 mL of 1.60 M of
Q58: The energy associated with a stretched spring