Multiple Choice
Which of the following chemical equations does not correspond to a standard molar enthalpy of formation?
A) Mg(s) + C(s) + 3/2 O2(g) → MgCO3(s)
B) C(s) + 1/2 O2(g) → CO(g)
C) N2(g) + O2(g) → 2 NO(g)
D) N2(g) + 2 O2(g) → N2O4(g)
E) H2(g) + 1/2 O2(g) → H2O( 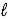 )
)
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: Exactly 149.6 J will raise the temperature
Q3: A bomb calorimeter has a heat capacity
Q4: The heat required to convert a solid
Q5: A chemical reaction in a bomb calorimeter
Q6: The following reaction of iron oxide with
Q7: The thermochemical equation for the combustion of
Q8: Determine the standard enthalpy of formation of
Q9: Heat capacity is defined as<br>A) the amount
Q10: Which of these physical changes would require
Q11: 44.0 g of ice at -20.0 °C