Multiple Choice
Aspirin (C9H8O4) is produced by the reaction of salicylic acid (M = 138.1 g/mol) and acetic anhydride (M = 102.1 g/mol) . C7H6O3(s) + C4H6O3( 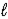 ) → C9H8O4(s) + C2H4O2(
) → C9H8O4(s) + C2H4O2( 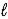 )
)
If you react 2.00 g C7H6O3 with 1.60 g C4H6O3,what mass of aspirin (M = 180.2 g/mol) can theoretically be obtained?
A) 0.40 g
B) 2.61 g
C) 2.82 g
D) 1.53 g
E) 3.60 g
Correct Answer:

Verified
Correct Answer:
Verified
Q60: A 30.00-mL sample of a weak monoprotic
Q61: Which of the following is the correct
Q62: A 2.288 g sample of a hydrocarbon
Q63: The numbers preceding the formulas in chemical
Q64: 2 Al(s)+ 6 HCl(aq)→ 2 AlCl<sub>3</sub>(aq)+ 3
Q66: Pure copper may be produced by the
Q67: Sulfur trioxide (SO<sub>3</sub>)is produced from the oxidation
Q68: Sulfur hexafluoride is produced by reacting elemental
Q69: A mass of 0.4113 g of an
Q70: What mass of Na<sub>2</sub>CO<sub>3</sub> is present in