Multiple Choice
If 0.1800 g of impure soda ash (Na2CO3) is titrated with 15.66 mL of 0.1082 M HCl,what is the percent purity of the soda ash? Na2CO3(aq) + 2 HCl(aq) → 2 NaCl(aq) + H2O( 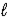 ) + CO2(g)
) + CO2(g)
A) 17.96%
B) 49.89%
C) 50.11%
D) 94.13%
E) 99.77%
Correct Answer:

Verified
Correct Answer:
Verified
Q39: Consider the fermentation reaction of glucose: C<sub>6</sub>H<sub>12</sub>O<sub>6</sub>
Q40: The reaction of 0.779 g K with
Q41: The empirical formula of styrene is CH.An
Q42: How many moles of sulfate ions are
Q43: Phosphorus trichloride,PCl<sub>3</sub>,can be produced from the reaction
Q45: A 1.078 g sample of a compound
Q46: The following equation: A = ε ×
Q47: When strongly heated,boric acid breaks down to
Q48: The pH of purified water (or of
Q49: Naphthalene,a hydrocarbon,has an approximate molar mass of