Short Answer
The following equation: A = ε × 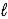 × C (where
× C (where 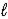 is path length and C is concentration)is known as the _____-Lambert law.
is path length and C is concentration)is known as the _____-Lambert law.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q41: The empirical formula of styrene is CH.An
Q42: How many moles of sulfate ions are
Q43: Phosphorus trichloride,PCl<sub>3</sub>,can be produced from the reaction
Q44: If 0.1800 g of impure soda ash
Q45: A 1.078 g sample of a compound
Q47: When strongly heated,boric acid breaks down to
Q48: The pH of purified water (or of
Q49: Naphthalene,a hydrocarbon,has an approximate molar mass of
Q50: What minimum mass of cobalt (II)nitrate must
Q51: A 1.850 g mixture of SrCO<sub>3</sub> and