Multiple Choice
Calculate the G ' of a redox reaction of pyruvate and hydrogen under standard conditions using the table below. 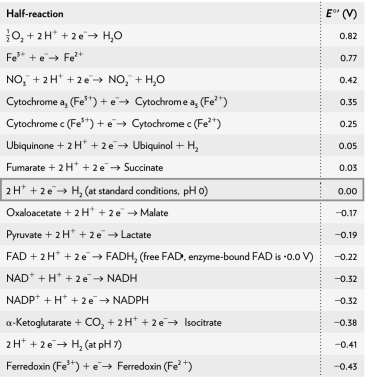
A) 18.33 kJ/mol
B) 36.66 kJ/mol
C) 54.99 kJ/mol
D) 73.32 kJ/mol
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q9: Which anaplerotic reaction balances the input of
Q11: What is the fate of oxaloacetate when
Q32: Describe the fate of NADH and FADH<sub>2</sub>
Q33: How is the pyruvate dehydrogenase reaction regulated?<br>A)
Q38: A high concentration of which molecule would
Q52: Use the figure below to answer the
Q53: Which enzyme of the citrate cycle
Q55: Identify the coenzyme that provides a reactive
Q57: Why is the <span class="ql-formula"
Q61: Which reactions in the citrate cycle produce