Essay
Use the figure below to answer the following questions.
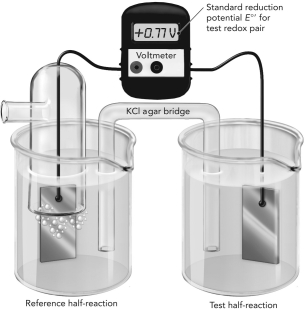 A. The electrochemical cell shown here is measuring the reduction potential of Fe2+/Fe3+ using a reference hydrogen half reaction. Write each half reaction on the figure and show the flow of electrons.
A. The electrochemical cell shown here is measuring the reduction potential of Fe2+/Fe3+ using a reference hydrogen half reaction. Write each half reaction on the figure and show the flow of electrons.
B. Do these oxidants have a higher or lower affinity for electrons compared with H+? Explain how you know this using information from the experiment.
Correct Answer:

Verified
A. The reference hydrogen half reaction ...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q11: What is the fate of oxaloacetate when
Q28: How would an increased level of acetyl-CoA
Q33: How is the pyruvate dehydrogenase reaction regulated?<br>A)
Q38: A high concentration of which molecule would
Q47: Which two enzymes in the citrate
Q48: The poison compound 1080 converts fluoroacetate
Q50: The reaction catalyzed by _ is
Q53: Which enzyme of the citrate cycle
Q56: Calculate the <span class="ql-formula" data-value="\Delta"><span
Q57: Why is the <span class="ql-formula"