Figure 6.4 -Figure 6.4 Illustratesvarious Aspects of the Free-Energy Change (ΔG)for the (ΔG)for
Multiple Choice
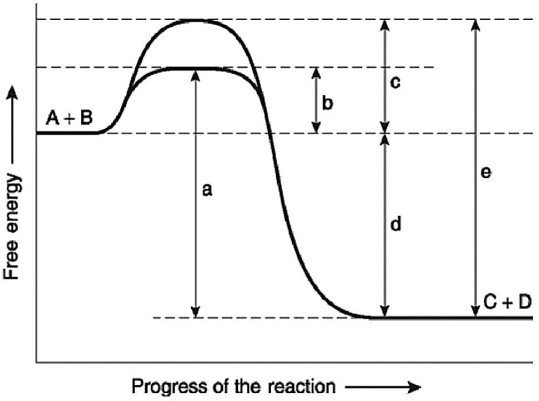 Figure 6.4
Figure 6.4
-Figure 6.4 illustratesvarious aspects of the free-energy change (ΔG) for the reaction A + B ↔ C + D.Which of the following best describes the forward reaction?
A) endergonic,∆G > 0
B) exergonic,∆G < 0
C) endergonic,∆G < 0
D) exergonic,∆G > 0
Correct Answer:

Verified
Correct Answer:
Verified
Q44: Which of the following reactions release energy?<br>A)endergonic
Q45: Which of the descriptions below is an
Q46: Hydrolysis of ATP releases energy,which ultimately results
Q47: When chemical,transport,or mechanical work is performed by
Q48: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7910/.jpg" alt=" Figure 6.1 -Which
Q50: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7910/.jpg" alt="
Q51: The ∆G for a particular enzyme-catalyzed reaction
Q52: A chemical reaction that has a positive
Q53: Cooperativity is a form of allosteric activation
Q54: Cells use the ATP cycle shown in