Multiple Choice
Carbon dioxide (CO2) is readily soluble in water,according to the equation CO2 + H2O ↔ H2CO3.Carbonic acid (H2CO3) is a weak acid.If CO2 is bubbled into a beaker containing pure,freshly distilled water,which of the following graphs correctly describes the results?
A) 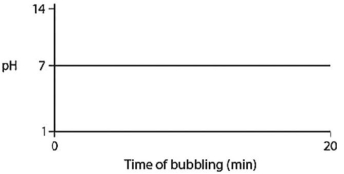
B) 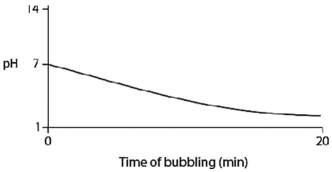
C) 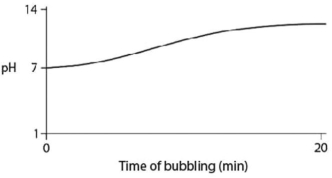
D) 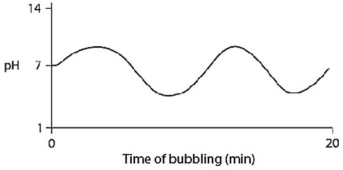
E) 
Correct Answer:

Verified
Correct Answer:
Verified
Q125: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7910/.jpg" alt=" Figure 2.6 -What
Q126: The atomic number of sulfur is 16,which
Q127: What is the hydroxyl ion (OH<sup>-</sup>)concentration of
Q128: In ammonium chloride salt (NH<sub>4</sub>Cl),the anion is
Q129: Buffers are substances that help resist shifts
Q130: The molar mass of glucose (C<sub>6</sub>H<sub>12</sub>O<sub>6</sub>)is 180
Q131: The chemical behavior of an atom depends
Q133: Research indicates that acid precipitation can damage
Q134: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7910/.jpg" alt=" Figure 2.1 Which
Q135: What is the pH of a 10<sup>-3</sup>