Multiple Choice
A solution is prepared by dissolving 0.23 mol of formic acid and 0.27 mol of sodium formate in water sufficient to yield 1.00 L of solution. The addition of 0.05 mol of HCl to this buffer solution causes the pH to drop slightly. The pH does not decrease drastically because the HCl reacts with the ________ present in the buffer solution. The 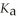 of formic acid is 1.36 × 10-3.
of formic acid is 1.36 × 10-3.
A) 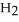 O
O
B) 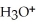
C) formate ion
D) formic acid
E) This is a buffer solution: the pH does not change at all upon addition of acid or base.
Correct Answer:

Verified
Correct Answer:
Verified
Q163: The titration of 25.0 mL of an
Q164: Which of the following is the correct
Q165: A 100.0 mL sample of 0.10 mol
Q166: Calculate the pH of a buffer that
Q167: Which of the following compounds will be
Q168: Which of the following is TRUE?<br>A) An
Q170: A 100.0 mL sample of 0.10 mol
Q171: What is the molar solubility of Mg(OH)<sub>2</sub>
Q172: A 1.00 L buffer solution is 0.250
Q173: Match the following.<br>-pH = 7<br>A)equivalence point of