Multiple Choice
What is the molar solubility of Mg(OH) 2 in a basic solution with a pH of 12.50? Ksp for Mg(OH) 2 is 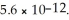
A) 1.8 × 10-10 mol L-1
B) 5.6 × 10-9 mol L-1
C) 2.4 × 10-6 mol L-1
D) 1.1 × 10-4 mol L-1
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q163: The titration of 25.0 mL of an
Q164: Which of the following is the correct
Q165: A 100.0 mL sample of 0.10 mol
Q166: Calculate the pH of a buffer that
Q167: Which of the following compounds will be
Q168: Which of the following is TRUE?<br>A) An
Q169: A solution is prepared by dissolving 0.23
Q170: A 100.0 mL sample of 0.10 mol
Q172: A 1.00 L buffer solution is 0.250
Q173: Match the following.<br>-pH = 7<br>A)equivalence point of