Multiple Choice
Calculate the pH of a solution that is 0.445 mol L-1 in sodium formate (HCOONa) and 0.265 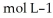 in formic acid (HCOOH) . The
in formic acid (HCOOH) . The 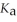 of formic acid is 1.77 ×
of formic acid is 1.77 × 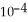 .
.
A) 4.860
B) 3.527
C) 10.023
D) 3.977
E) 3.752
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q83: Determine the molar solubility of Fe(OH)<sub>2 </sub>in
Q84: For a monoprotic weak acid titrated with
Q85: A 100.0 mL sample of 0.10 mol
Q86: A 100.0 mL sample of 0.20 mol
Q87: Determine the molar solubility of AgI in
Q89: What is the pH of a buffer
Q90: What is the pH of a solution
Q92: What is the pH of a solution
Q93: Which of the following acids (listed with
Q173: Match the following.<br>-pH = 7<br>A)equivalence point of