Multiple Choice
What is the pH of a solution made by mixing 10.00 mL of 0.10 mol L-1 acetic acid with 10.00 mL of 0.10 mol L-1 KOH? Assume that the volumes of the solutions are additive. Ka = 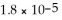 for CH3COOH.
for CH3COOH.
A) 5.28
B) 7.00
C) 8.72
D) 10.02
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q87: Determine the molar solubility of AgI in
Q88: Calculate the pH of a solution that
Q89: What is the pH of a buffer
Q90: What is the pH of a solution
Q93: Which of the following acids (listed with
Q95: A 25.0 mL sample of 0.150 mol
Q96: A 100.0 mL sample of 0.10 mol
Q97: Calculate the pH of a solution that
Q128: Which of the following compounds will have
Q173: Match the following.<br>-pH = 7<br>A)equivalence point of