Multiple Choice
A 25.0 mL sample of 0.150 mol L-1 hydrofluoric acid is titrated with a 0.150 mol L-1 NaOH solution. What is the pH before any base is added? Hydrofluoric acid is monoprotic. The 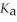 of hydrofluoric acid is 3.5 × 10-4.
of hydrofluoric acid is 3.5 × 10-4.
A) 2.33 × 10-3
B) 2.63
C) 3.46
D) 7.07 × 10-3
E) 2.15
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q90: What is the pH of a solution
Q92: What is the pH of a solution
Q93: Which of the following acids (listed with
Q96: A 100.0 mL sample of 0.10 mol
Q97: Calculate the pH of a solution that
Q98: When titrating a monoprotic strong acid with
Q99: Identify the expression for the solubility product
Q100: Calculate the pH of a solution formed
Q128: Which of the following compounds will have
Q173: Match the following.<br>-pH = 7<br>A)equivalence point of