Multiple Choice
A solution of methanol and water has a vapour pressure of 248 Torr. What would you predict as the mole fractions of each component, assuming ideal behaviour? The vapour pressures of methanol and water are 256 Torr and 55.3 Torr, respectively.
A) 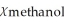 = 0.990,
= 0.990,  = 0.010
= 0.010
B) 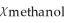 = 0.940,
= 0.940,  = 0.060
= 0.060
C) 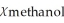 = 0.960,
= 0.960,  = 0.040
= 0.040
D) 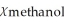 = 0.910,
= 0.910,  = 0.090
= 0.090
E) 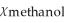 = 0.900,
= 0.900,  = 0.100
= 0.100
Correct Answer:

Verified
Correct Answer:
Verified
Q57: Determine the molality of a solution prepared
Q58: What is the expected van't Hoff factor
Q59: At 20 °C, a 0.389 mol L<sup>-1</sup>
Q60: A nonelectrolyte compound with a molar mass
Q61: At 20 °C, a 2.72 mol L<sup>-1</sup>
Q63: Choose the aqueous solution with the lowest
Q64: Determine the molality of an aqueous solution
Q65: Choose the situation below that would result
Q66: Calculate the mole fraction of total ions
Q67: Identify the major force between acetone and