Multiple Choice
At 20 °C, a 0.389 mol L-1 aqueous solution of ammonium chloride has a density of 1.0064 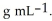 What is the mass % of ammonium chloride in the solution? The formula weight of
What is the mass % of ammonium chloride in the solution? The formula weight of 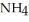 Cl is
Cl is 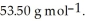
A) 0.732%
B) 2.59%
C) 0.395%
D) 0.387%
E) 2.07%
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q54: Calculate the vapour pressure of pure water
Q55: Determine the freezing point depression of a
Q56: Calculate the freezing point of a solution
Q57: Determine the molality of a solution prepared
Q58: What is the expected van't Hoff factor
Q60: A nonelectrolyte compound with a molar mass
Q61: At 20 °C, a 2.72 mol L<sup>-1</sup>
Q62: A solution of methanol and water has
Q63: Choose the aqueous solution with the lowest
Q64: Determine the molality of an aqueous solution