Multiple Choice
Vanadium crystallizes in a body-centred cubic structure and has an atomic radius of 131 pm. Determine the density of vanadium if the edge length of a bcc structure is 4r/ 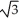 .
.
A) 3.06 g cm-3
B) 12.2 g cm-3
C) 6.11 g cm-3
D) 2.77 g cm-3
E) 8.46 g cm-3
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q10: Choose the substance with the highest boiling
Q11: The enthalpy change for converting 10.0 g
Q12: Identify the term used to describe the
Q12: Choose the compound that exhibits hydrogen bonding
Q14: What type of intermolecular force causes the
Q16: Give the coordination number for a body-centred
Q17: Based on the figure above, the boiling
Q43: Choose the pair of substances that are
Q61: Which type of bonding does Sr form
Q69: Choose the substance with the lowest boiling