Multiple Choice
The enthalpy change for converting 10.0 g of ice at -25.0 °C to water at 80.0 °C is ________ kJ. The specific heats of ice, water, and steam are 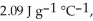
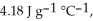 and
and 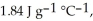 respectively. For
respectively. For 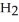 O,
O, 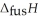 = 6.01 kJ mol-1, and
= 6.01 kJ mol-1, and 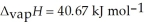 .
.
A) 7.21
B) 6.16
C) 3870
D) 63.97
E) 26.46
Correct Answer:

Verified
Correct Answer:
Verified
Q2: Describe the difference between the conduction band
Q7: Which of the following substances would you
Q10: Choose the substance with the highest boiling
Q12: Choose the compound that exhibits hydrogen bonding
Q12: Identify the term used to describe the
Q13: Vanadium crystallizes in a body-centred cubic structure
Q14: What type of intermolecular force causes the
Q16: Give the coordination number for a body-centred
Q61: Which type of bonding does Sr form
Q69: Choose the substance with the lowest boiling