Multiple Choice
The enthalpy change for converting 1.00 mol of ice at -60.0°C to water at 80.0°C is  The specific heats of ice, water, and steam are
The specific heats of ice, water, and steam are 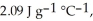
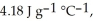 and
and 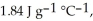 respectively. For
respectively. For 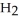 O,
O, 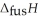 = 6.01 kJ mol-1, and
= 6.01 kJ mol-1, and 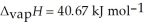 .
.
A) 9.77
B) 14.29
C) 8282
D) 13.17
E) 48.95
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Why is the Δ<sub>vap</sub>H higher than Δ<sub>fus</sub>H<sub>
Q2: Determine the vapour pressure (in mbar) of
Q2: Describe the difference between the conduction band
Q5: Which of the following forms an ionic
Q7: Which of the following substances would you
Q10: Choose the substance with the highest boiling
Q11: The enthalpy change for converting 10.0 g
Q12: Choose the compound that exhibits hydrogen bonding
Q69: Identify the type of solid for argon.<br>A)
Q69: Choose the substance with the lowest boiling