Multiple Choice
According to the following thermochemical equation, what mass of HF (in g) must react to produce 345 kJ of energy? Assume excess SiO2. SiO2(s) + 4HF(g) → SiF4(g) + 2H2O(l) 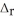 H° = -184 kJ
H° = -184 kJ
A) 42.7 g
B) 37.5 g
C) 150. g
D) 107 g
E) 173 g
Correct Answer:

Verified
Correct Answer:
Verified
Q6: How much heat is absorbed when 50.00
Q7: Calculate the final temperature of a 38.1
Q8: A sample of copper absorbs 43.6 kJ
Q9: Which of the following processes is endothermic?<br>A)
Q10: Use the standard reaction enthalpies given below
Q12: Calculate the change in internal energy (ΔU)
Q13: Match the following.<br>--ΔU<br>A)energy flows out of the
Q14: Match the following.<br>-energy associated with the motion
Q15: Match the following.<br>--w<br>A)energy flows out of the
Q16: Sodium metal reacts with water to produce