Multiple Choice
The anhydride formed between a carboxylic acid and a phosphate (Figure 3-45A) is a high-energy intermediate for some reactions in which ATP is the energy source.Arsenate can also be incorporated into a similar high-energy intermediate in place of the phosphate (Figure 3-45B) .Figure 3-45C shows the reaction profiles for the hydrolysis of these two high-energy intermediates.What is the effect of substituting arsenate for phosphate in this reaction? (A) 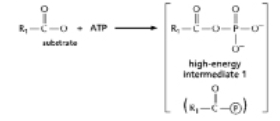
(B) 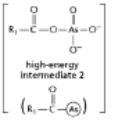
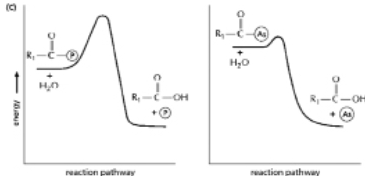
Figure 3-45
A) It forms a high-energy intermediate of lower energy.
B) It forms a high-energy intermediate of the same energy.
C) It decreases the stability of the high-energy intermediate.
D) It increases the stability of the high-energy intermediate.
Correct Answer:

Verified
Correct Answer:
Verified
Q14: Oxidation is the process by which oxygen
Q15: The equilibrium constant for complex formation between
Q16: For each of the pairs A-D
Q17: In general, there is a positive change
Q18: The graph in Figure 3-34 illustrates the
Q20: Your body extracts energy from the food
Q21: In the case of a simple conversion
Q22: You are studying a biochemical pathway
Q23: At first glance, it may seem that
Q24: Indicate whether the following statements are TRUE