Short Answer
The combustion of carbon disulfide in the presence of excess oxygen yields carbon dioxide and sulfur dioxide:
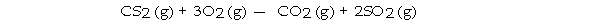
The combustion of 15 g of CS2 in the presence of excess oxygen yields g of SO2.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q8: A compound was found to contain 90.6%
Q73: How many atoms of nitrogen are in
Q74: What mass in grams of hydrogen is
Q75: There are _ sulfur atoms in 25
Q76: Of the reactions below, which one is
Q77: A compound contains 40.0% C, 6.71% H,
Q79: Carbon dioxide called a greenhouse gas because
Q81: One million argon atoms is mol of
Q82: One mole of contains the largest number
Q83: GeF<sub>3</sub>H is formed from GeH<sub>4</sub><sub> </sub>and GeF<sub>4</sub><sub>