Multiple Choice
What mass in grams of hydrogen is produced by the reaction of 4.73 g of magnesium with 1.83 g of water? 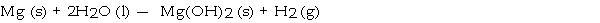
A) 0.0485
B) 0.219
C) 0.102
D) 0.0162
E) 0.204
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q69: Calculate the percentage by mass of chlorine
Q70: How many moles of carbon dioxide are
Q71: What is the maximum mass in grams
Q72: Under appropriate conditions, nitrogen and hydrogen undergo
Q73: How many atoms of nitrogen are in
Q75: There are _ sulfur atoms in 25
Q76: Of the reactions below, which one is
Q77: A compound contains 40.0% C, 6.71% H,
Q78: The combustion of carbon disulfide in the
Q79: Carbon dioxide called a greenhouse gas because