Multiple Choice
The mass of a proton is 1.00728 amu and that of a neutron is 1.00867 amu. What is the binding energy per nucleon (in J) of a 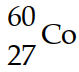 nucleus? (The mass of a cobalt- 60 nucleus is 59.9338 amu.)
nucleus? (The mass of a cobalt- 60 nucleus is 59.9338 amu.)
A) 7.009 × 10- 14
B) 2.487 × 10- 12
C) 1.368 × 10- 12
D) 9.432 × 10- 13
E) 3.039 × 10- 12
Correct Answer:

Verified
Correct Answer:
Verified
Q32: The missing product from this reaction is
Q33: The mass of a proton is 1.00728
Q34: The three radioactive series that occur in
Q35: In the nuclear transmutation represented by <img
Q36: Which one of the following forms of
Q38: Which one of the following devices converts
Q39: The main scientific difficulty in achieving a
Q40: What is emitted in the nuclear transmutation,<img
Q41: The only element with no neutrons is
Q42: The half- life for beta decay of