Multiple Choice
The mass of a proton is 1.00728 amu and that of a neutron is 1.00867 amu. What is the mass defect (in amu) of a 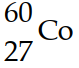 nucleus? (The mass of a cobalt- 60 nucleus is 59.9338 amu.) .?
nucleus? (The mass of a cobalt- 60 nucleus is 59.9338 amu.) .?
A) 0.0662
B) 0.5405
C) 27.7830
D) 0.4827
E) 0.5489
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q28: What is the largest number of protons
Q29: A freshly prepared sample of curium- 243
Q30: Which one of the following requires a
Q31: The alpha decay of what isotope of
Q32: The missing product from this reaction is
Q34: The three radioactive series that occur in
Q35: In the nuclear transmutation represented by <img
Q36: Which one of the following forms of
Q37: The mass of a proton is 1.00728
Q38: Which one of the following devices converts